Therapeutic Antibodies from Human Donors
Therapeutic antibodies and their derivatives (antibody fragments, ADC or Antibody Drug Conjugate conjugates, etc.) are today the most dynamic segment of the biopharmaceutical market. Indeed, the properties of antibodies in terms of specificity, affinity and stability make these molecules the first choice therapeutic agents.
In order to reduce their immunogenicity and increase their efficacy, therapeutic antibodies have gradually evolved from a murine format to recombinant formats containing ever-increasing proportions of human sequences. Thus, chimeric antibodies were the first recombinant antibodies generated but quite quickly supplanted by humanized antibodies and fully human antibodies. The latter are now the most popular therapeutic antibodies in marketing authorization applications.
BIOTEM does not claim any intellectual properties nor any other rights on the developed antibodies.
- Fully exploiting human immune response
- Robust screening system
- Validation of intermediate and final clones by the client.
Project
Specifications
1. Project Specifications
For each project, our team explores with the client the challenges of the project and defines together the specifications.
- Target of interest: membrane protein, peptide, small molecule, post-translational modifications, etc.
- Special features: Human donors, available material (screening antigen …), specificity, deadlines, etc.
Taking into account these multiple parameters, BIOTEM proposes tailor-made strategies to meet the client's needs and guarantee the success of the project.
Donor
Selection
2. Donor Selection
BIOTEM's strategy to generate 100% human monoclonal antibodies consists in building an immune library from biological materials derived from donors (in this case, often patients) naturally immunized against a target of interest (a virus, a bacteria...).
The identification of potential donors which have been generating a specific immune response is one of the major objective of the preliminary phase.
Library &
Phage Display
3. Library & Phage Display
Unlike naive or synthetic libraries, BIOTEM proposes the construction of an immune library, in scFv format, focused on the target of interest combined with high throughput screening (or bio-panning) by phage display. This optimizes the chances of success in obtaining high affinity and high specificity antibodies.- Selection of a large number of scFv candidates (VH+VL only)
- Optimized screening with different stringency conditions (direct, competitive, subtractive, etc.)
- Immediate and automatic access to sequences (single clones - redundancy analysis)
- Production, Purification and Characterization of candidates (reactivity, affinity, etc.).
At the end of this phase the client will have the opportunity to test the scFv in his application and select the best candidates for the reformatting phase in full Ig or other formats.
Reformatting &
Engineering
4. Reformatting
This step allows the transition from scFv to full immunoglobulin format (or orther formats: scFv-Fc, etc.) thanks to a fully integrated platform for the production of recombinant antibodies in mammalian cells (CHO). Learn more
- Several Isotypes
- Sequence Optimization
- Antibody Engineering (Isotype, mutations, Fc-fusion protein, Bispecific antibodies, Fab, etc.)
- Antibody Variants
- Production & Purification (pilot study).
The CLIENT will decide which candidates to produce in large scales.
Production &
purification
5. Production & purification
Large scale productions are available with our CHO cell Platform. Learn more
- From milligram to several grams
- Low endotoxin Conditions (< 10 EU/mg ; even < 1 EU/mg)
- Serum free System
- Antibody Engineering
- Quality Control (concentration, purity, affinity, aggregation, stability, etc.)
Project
Specifications
1. Project Specifications
For each project, our team explores with the client the challenges of the project and defines together the specifications.
- Target of interest : membrane protein, peptide, small molecule, post-translational modifications, etc.
- Special features : Human donors, Available material (screening antigen …), specificity, deadlines, etc.
Taking into account these multiple parameters, BIOTEM will propose tailor-made strategies to meet the client's needs and guarantee the success of the project.
Donor
Selection
2. Donor Selection
The strategy handled by BIOTEM to generate fully human monoclonal antibodies is based on the immortalisation of memory B cells. These cells express naturally, on their surface, specific Human antibodies targeting a virus, a bacteria, ...
The identification of potential donors which have been generating a specific immune response is one of the major objective of the preliminary phase.
Enrichment
& Immortalization
3. Enrichment & Immortalization
- Selection: PBMCs (Peripheral Blood Mononuclear Cells), containing the memory B cells, are extracted from whole blood using a density gradient method. BIOTEM has developed different enrichment strategies which improve the activation and immortalisation phases of the project
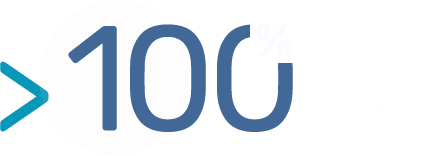
- Cell Activation: The activation of the selected cells using specific media achieves a high immortalisation rate (close to 100 %).This phase, performed in vitro, is based on an unique process and know-how developed by BIOTEM
- Immortalization EBV (Epstein Barr Virus): The immortalization is induced by contact between activated cells and viral particles (also known as virions). Subsequently, the memory B cells differentiate into LCLs (Lymphoblastoïd Cell Line) which are characterised by high proliferation and antibody secretion.
Screening
& Cloning
4. Screening & Cloning
The screening phase enables the selection of LCLs secreting antibodies specific to the antigen. The methods used are adapted for each project according to the client's specifications (conventional, competitive, multiple screening, etc.). The positive clones from the screening are then confirmed (polyclonal stage). The candidates selected by the client are cloned and stabilized (limit dilution). This step is necessary to obtain monoclonal lines. A validated cell bank (cryotubes) will be carried out, including a viability test.
Production
& purification
5. Production & purification
BIOTEM offers different solutions for the production and purification of antibodies, from pilot batch to large-scale. Low Bovine IgG and Low Endotoxin (< 10 EU/mg) production conditions can also be offered. Learn more
- In vitro production: Flask, HYPERFlask®, CELLine, Bioreactors, etc.
- In vivo production: Production in liquid ascites
- Purifications : Affinity chromatography, precipitation, size exclusion, etc.
Additional services such as antibody characterization (sequencing, affinity, etc.) or modification (coupling, fragmentation, etc.) are also available.


Pour accéder au document, veuillez remplir le formulaire