Monoclonal Antibody for Immunohistochemistry (IHC)
Immunohistochemistry (IHC) is an analytical method widely used in anatomopathology. It requires extensive sample processing which can significantly modify the target of interest. Many commercial antibodies are available and validated for IHC on frozen sections but are not always adapted to other sample processing conditions and/or suffer from a lack of specificity.
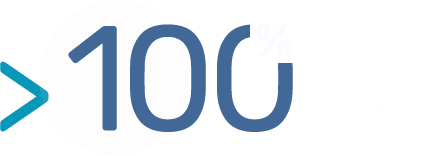
To address this issue, BIOTEM now offers a platform entirely dedicated to the development of functional monoclonal antibodies in IHC with a particularly high success rate for paraffin-embedded specimens (formalin-fixed, paraffin-embedded slides or FFPE slides) thanks to the design / preparation of immunogens and screening antigens.


BIOTEM does not claim any intellectual properties nor any other rights on the developed antibodies.
- IHC Peptide Profiler® : Exclusive program developed by BIOTEM for the design of optimized peptide
- IHC Formulation : Designed to subtly modify proteins in order to mimic the targeted epitopes
- Antibody Characterization at each stage
An increasingly widespread technology, phage display enables the generation of recombinant format antibodies derived from an immune library constructed from immunized animals. This approach offers the possibility of testing a large number of clones thanks to a high throughput screening and allows access to the sequences from the first phases.
Project
Specifications
For each project, our team explores with the client the challenges of the project and defines the specifications together.
- The target of interest: membrane protein, peptide, small molecule, post-translational modifications, etc.
- Applications: IHC on frozen or paraffin-embedded sections, etc.
- Specifications: Available material (immunogen / antigen / cells / tissues), specificity, deadlines, etc.
Taking into account these multiple parameters, BIOTEM proposes tailor-made strategies to meet the client's needs and guarantee the success of the project.
Immunogen Design
& Preparation
2. Immunogen & Immunization
Immunogen Design & Preparation- IHC Peptide Profiler®: This exclusive program developed by BIOTEM allows the design of the most relevant peptide immunogens to be used in the generation of IHC-reactive monoclonal antibodies. A complete analysis of the target (sequence, three-dimensional structure, modifications...) combined with a multiparametric study – according to 9 specific criteria – of the consequences of antigen treatments in IHC allows the selection and ranking of the best peptide candidates.
- IHC Protein Formulations : Available only from BIOTEM, the IHC-specific antigen formulations that are designed to subtly modify proteins in order to obtain immunogens that mimic the targeted epitopes as they appear on IHC paraffin sections. Such a complementary approach to the IHC Peptide Profiler® allows a broadening of the eventual diversity in generated antibodies.
Depending on the client's specifications, BIOTEM has a wide range of proven immunization strategies to obtain high affinity and specificity antibodies. Specific methods are developed to ensure that the appropriate immune response is achieved. At the end of this phase BIOTEM will be able to offer a contract with guaranteed results. The client will also have the possibility to test the sera in his application.
Library &
Phage Display
3. Librairy & Phage Display
- Selection of a large number of scFv candidates (VH+VL only)
- Optimized screening with different stringency conditions (direct, competitive, subtractive, etc.)
- Immediate access to sequences (single clones - redundancy analysis)
- Antibody Engineering (Isotype, mutations, Fc-fusion protein, Bispecific antibodies, Fab, etc.)
- Variants (Chimerization, humanization, etc.)
- Production, Purification and Characterization of candidate (reactivity, affinity, etc.).
At the end of this phase the client will have the opportunity to test the scFv and select the best candidates for the reformatting phase in full Ig format.
Reformatting
& Engineering
4. Reformatting
This step allows the transition from scFv to full immunoglobulin format (or orther formats : scFv-Fc) thanks to a fully integrated platform for the production of recombinant antibodies in mammalian cells (CHO). More info
- Several Isotypes
- Sequence Optimization
- Antibody Engineering (Isotype, mutations, Fc-fusion protein, Bispecific antibodies, Fab, etc.)
- Variants (Chimerization, humanization,etc.)
- Production & Purification (pilot study).
The CLIENT will decide which candidates to produce in large scales.
Production &
purification
5. Production & purification
Large scale productions are available with our CHO cell Platform. More info
- From milligram to several grams
- Low endotoxin Conditions
- Serum free System
- Quality Control (concentration, purity, affinity, aggregation, stability, etc.)
An antibody development project using hybridoma technology is organized around four crucial phases, after which the client has the opportunity to test the material and decide on the continuation of the project (Go / No Go Phases). During these different stages, BIOTEM implements optimized operational management of projects with strict quality controls and personalized support throughout the program.
Project
Specifications
1. Project Specifications
For each project, our team explores with the client the challenges of the project and defines the specifications together.
- The target of interest: membrane protein, peptide, small molecule, post-translational modifications, etc.
- Applications: IHC on frozen or paraffin-embedded sections (FFPE), etc.
- Specifications: Available material (immunogen / antigen / cells / tissues), specificity, deadlines, etc.
Taking into account these multiple parameters, BIOTEM proposes tailor-made strategies to meet the client's needs and guarantee the success of the project.
Immunogen
& Immunization
2. Immunogen & Immunization
Immunogen Design & Preparation
- IHC Peptide Profiler®: This exclusive program developed by BIOTEM allows the design of the most relevant peptide immunogens to be used in the generation of IHC-reactive monoclonal antibodies. A complete analysis of the target (sequence, three-dimensional structure, modifications...) combined with a multiparametric study – according to 9 specific criteria – of the consequences of antigen treatments in IHC allows the selection and ranking of the best peptide candidates.
- IHC Protein Formulations: Available only from BIOTEM, the IHC-specific antigen formulations that are designed to subtly modify proteins in order to obtain immunogens that mimic the targeted epitopes as they appear on IHC paraffin sections. Such a complementary approach to the IHC Peptide Profiler® allows a broadening of the eventual diversity in generated antibodies.
Fusion &
Screening
3. Fusion & Screening
Depending on the Phase I results, the client will decide whether to launch subsequent phase or not. With its expertise, BIOTEM offers optimized screening methods to identify the best hybridomas (oligoclonal stage).
At each stage, all the biologics (sera, supernatants, recombinant scFv, etc.) will be evaluated and characterized.Cloning
Hybridomas
4. Cloning
The selected hybridomas are then stabilized by limiting dilution. This step is necessary to obtain monoclonal lines. A validated cell bank (vials) will be carried out, including a viability test.
The vials can be transferred to the client or stored in secure containers at BIOTEM for future productions (see additional services). (Learn more)
Production &
purification
5. Production & Purification
BIOTEM offers different solutions for the production and purification of antibodies, from pilot batch to large-scale. Low Bovine IgG and Low Endotoxin (< 10 EU/mg) production conditions can also be offered.
- In vitro Production : Flask, HYPERFlask®, CELLine, Bioreactors, etc.
- In vivo Production : Production in liquid ascites
- Purifications : Affinity chromatography, precipitation, size exclusion, etc. Learn more
Additional services such as antibody characterization (sequencing, affinity, etc.) or modification (coupling, fragmentation, etc.) are also available.

To access the document, fill the form