Mouse and Rat Monoclonal Antibody Production
Phage Display & Hybridoma Technologies
BIOTEM has developed a fully recognized know-how in the discovery, the generation and the production of murine monoclonal antibodies.
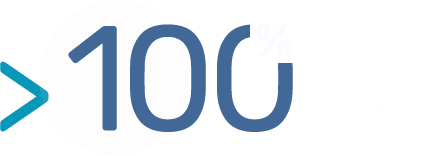
In addition since 1980 and with more than 150 projects per year, the team has a well established expertise in challenging targets (membrane protein, small molecule, conserved target, etc.).
Monoclonal antibody development and production is mainly based on 2 technologies: Hybridoma or Phage Display (recombinant antibodies).
Each project is based on several crucial phases at the end of which the customer has the opportunity to test the biological material and decide to continue the project (Go/ No Go Milestones).
NEW ESSENTIAL OFFER! You want to develop your own hybridomas on a tight budget?
Contact our team to discuss your project and check your eligibility!
BIOTEM does not claim any intellectual properties or any other rights on the developed antibodies.
During the program, BIOTEM implements :
- Optimized Project Management
- Strict Quality Controls
- Personalized Support
- Client Testing throughout the Program (Go/ No Go Milestones)
Project
Specifications
1. Project Special Features
For each case, our team explores with the client the challenges of the project and defines the specifications together.
- Target of interest : membrane protein, peptide, small molecule, post-translational modifications, etc.
- Applications : ELISA, WB, FACS, IF, IHC, IP, functional tests, diagnostic or therapeutic applications, etc.
- Special features : Available material, specificity, deadlines, etc.
Taking into account these multiple parameters, BIOTEM proposes tailor-made strategies to meet the client's needs and guarantee the success of the project.
Immunization
mouse & rat
2. Immunization
To meet each precise demand and client’s specifications, BIOTEM relies on a wide range of proven immunization strategies to obtain high affinity and specificity antibodies. Specific methods are developed to ensure that the appropriate immune response is achieved. The client will also have the possibility to test the sera in his/her application. At the end of this phase BIOTEM will be able to propose a contract with guaranteed results.
- Protein Immunization : From the production of the recombinant protein (transient transfection in CHO) to its preparation for immunization.
- Peptide Immunization : BIOTEM has the experience and know-how to optimally integrate all the parameters and constraints of immunogenic peptides design. Immunization with peptides is a relevant strategy for the generation of antibodies directed against selected epitopes. This approach also reduces the need for sophisticated protein preparations for immunization.
- Non-exhaustive list
Library &
Phage Display
3. Library Construction & Phage Display
Unlike naive or synthetic libraries, BIOTEM proposes the construction of an immune library, in scFv format, focused on the target of interest combined with high throughput antibody screening (or bio-panning) by phage display. This optimizes the chances of success in obtaining high affinity and high specificity antibodies.
- Selection of a large number of scFv candidates (VH + VL only)
- Optimized screening with different stringency conditions (direct, competitive, subtractive, etc.)
- Immediate and automatic access to sequences (single clones - redundancy analysis)
- Production, Purification and Characterization of candidates (reactivity, affinity, etc.)
At the end of this phase the client will have the opportunity to test the scFv and select the best candidates for the reformatting phase in full Ig format.
Reformatting
& Engineering
4. Reformatting & Antibody Engineering
This step allows the transition from scFv to full immunoglobulin format (or orther formats : scFv-Fc, etc.) thanks to a fully integrated platform for recombinant antibody production in mammalian cells (CHO). Learn more
- Several Isotypes & Species
- Sequence Optimization
- Antibody Engineering (Isotype, mutations, Fc-fusion protein, Bispecific antibodies, Fab, etc.)
- Variants (Chimerization, humanization,etc.)
- Recombinant Antibody Production & Purification (pilot study).
Sending of the pilot lot for client testing and selecting the final candidate(s) for large scale production.
Production &
purification
5. Antibody Production & Purification
BIOTEM provides its platform for recombinant antibody production by transfection into CHO cells for large-scale production. Learn more
- From milligram to several grams
- « Low endotoxin » Conditions(< 10 EU/mg ; down to < 1 EU/mg)
- Serum-free System
- Quality Control (concentration, purity, affinity, aggregation, stability, etc.)
Project
Specifications
1. Project Special Features
For each case, our team explores with the client the challenges of the project and together, they define the specifications.
- The target of interest : membrane protein, peptide, small molecule, post-translational modifications, etc.
- Applications : ELISA, WB, FACS, IF, IHC, IP, functional tests, diagnostic or therapeutic application, etc.
- Specifications : Available material, specificity, deadlines, etc.
Taking into account these multiple parameters, BIOTEM proposes tailor-made strategies to meet the client's needs and guarantee the success of the project.
Immunization
mouse & rat
2. Immunization
To meet each precise demand and client’s specifications, BIOTEM relies on a wide range of proven immunization strategies to obtain high affinity and specificity antibodies. Specific methods are developed to ensure that the appropriate immune response is achieved. The client will also have the possibility to test the sera in his/her application. At the end of this phase BIOTEM will be able to propose a contract with guaranteed results.
- Protein Immunization : From the production of the recombinant protein (transient transfection in CHO) to its preparation for immunization
- Peptide Immunization : BIOTEM has the experience and know-how to optimally integrate all the parameters and constraints of the design of immunogenic peptides. Immunization with peptides is a relevant strategy for the generation of antibodies directed against selected epitopes. This approach also reduces the need for sophisticated protein preparations for immunization (see also IHC Peptide Profiler®).
- R.A.D® Protocol (Exclusive) : Rapid Antibody Development allows the fast development of hybridomas in less than a month (immunization, fusion and screening included) and offers the possibility to work on highly conserved targets.
- S.S.I. Protocol (Exclusive) : Small Size Immunogen, optimized for small targets (< 30 kDa).
- Subtractive immunization : These techniques are particularly useful for the generation of antibodies against epitopes that are poorly immunogenic and/or sharing a high degree of homology
- Other - Small molecules (haptens), Genetic, cellular or in vitro immunization : Suitable solutions when immunogens are difficult to obtain or in the case of toxic targets.
Fusion &
Screening
3. Lymphocyte Hybridization & Hybridoma Screening
Depending on the Phase I results, the client will decide whether to launch subsequent phase or not (Go / No-Go). With its expertise, BIOTEM offers optimized screening methods to identify the best hybridomas (oligoclonal stage).
- Fusion/ Lymphocyte Hybridization: Fusion of the lymphocytes with myeloma cells to produce hybridomas (immortalization)
- Antibody Screening: A crucial step in the project to isolate reactive and specific clones. The methods used are adapted for each project according to the client's specifications (standard, competitive, multiple screening, etc.). Several screening rounds are generally carried out
- Confirmations: The positive clones from the screening are then retested. At this stage the client will have the possibility to test the positive clones
- Cell Bank: After the tests performed by the client, the selected hybridomas are secured.
Cloning
Hybridomas
4. Hybridoma Cloning
The selected hybridomas are then stabilized by limiting dilution. This step is necessary to obtain monoclonal lines. A validated cell bank (cryogenic vials) will be carried out, including a viability test.
The client will have the option to receive intermediate supernatants to test in his/her application.
The cryogenic vials can be transferred to the client or stored in secure containers at BIOTEM for future productions. (Learn more).
Production &
purification
5. Antibody Production & Purification
BIOTEM offers different solutions for the production and purification of antibodies, from pilot batch to large-scale. Low Bovine IgG and Low Endotoxin (< 1 EU/mg) production conditions can also be offered.
- In vitro Production : Flask, HYPERFlask®, CELLine, Bioreactors, etc.
- In vivo Production : Production in ascitic fluids
- Purifications : Affinity chromatography, precipitation, size exclusion, etc. Learn more
Additional services such as antibody characterization (sequencing, affinity, etc.) or modification (coupling, fragmentation, etc.) are also available.


To access the document, please fill the form