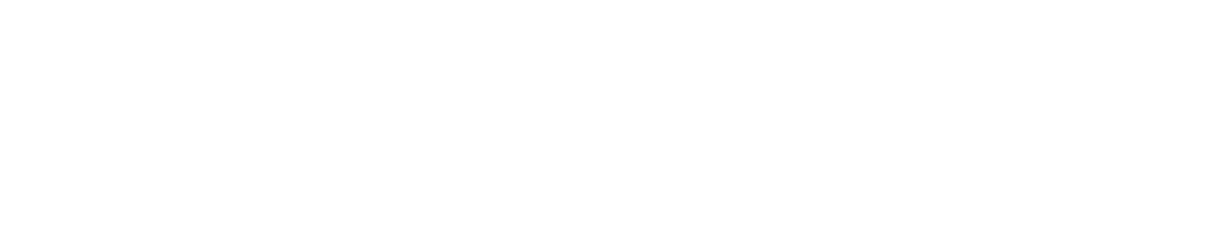
ELISA & Lateral Flow Production
In addition to its solutions for the custom development of ELISA and LFIA (Lateral Flow Immunoassay) tests, BIOTEM provides its clients with a fully-equipped, integrated and adjustable platform for the industrial production / manufacturing of kits.
From the production of research prototypes and validation batches, to industrialization studies and large-scale production, the teams support clients in their process to supply high-quality kits to meet their needs.
The production platform is also available to customers to complement existing capacities, or to outsource specific production steps.
Thanks to the ISO 13485:2016 certification (see Quality Policy), BIOTEM will be able to provide the documents required by the client to complete the files necessary to market in vitro diagnostic medical devices (IVDMD) in Europe.
Our services are available for all types of applications:
Human health, Animal health, Industrial quality control, Agriculture, Biosecurity, Drugs, Environment, etc.

1. Project specifications
The BIOTEM team works with the client to fully understand the project’s challenges and define the specifications:
- Type of test: ELISA (96-well plates) or LFIA rapid test (strip or cassette)
- Material available (antibodies, antigens, plates, solid phases, etc.)
- Sourcing of commercial references
- Available material (antibodies, antigens, samples, etc.)
- Transfer study
- Production process
- Quantities required and forecast
2. Transfer Study
If a prototype is available or a test is already on the market, BIOTEM can set up a transfer study. This study enables the current test to be transposed identically using BIOTEM equipment. This new batch will then be tested by BIOTEM and by the client before moving on to the next stage.
At the end of the transfer study, a performance diagnosis and recommendations/optimizations can be implemented. BIOTEM will also present a production plan detailing the operational procedures to be implemented according to the client’s specifications.
3. Test Optimization
BIOTEM optimizes prototypes initially developed. During this phase several validations of the prototypes are undertaken in order to ensure that the development is in line with the project specifications.
BIOTEM produces and sends to the client a batch of the new prototype(s) for evaluation and/or validation.
4. Validation
BIOTEM and the client jointly carry the formal evaluation (laboratory or field trials) of the performance and the several characteristics of the test. This validation phase is performed to meet the client’s specifications :
- Limit of detection
- Linearity / Parallelism
- Specificity
- Precision
- Inter- and Intra-assay reproducibility
- Robustness
- Stability studies of the test and its components
5. Production & Industrialization
To complete the project, BIOTEM proposes several options to the client :
- Transfer of technology to the client or client’s manufacturer
- Industrialization and batch production
BIOTEM offers a fully integrated and scalable manufacturing platform for the industrial production ELISA and Lateral Flow (strips/ cassettes) kits. A semi-automated platform based in France, certified ISO 13485:2016 for IVD with state-of-the-art equipment, allows a perfect adaptation to all needs in terms of capacity and lead times.
- Flexible and Robust Process
- Fully Integrated Manufacturing Platform based in France (Apprieu)
- Personalized Assistance in all steps of the production
- Dedicated Equipment and Teams
- Small, Medium and Large Scale Production (24/7)
- Quality Certifications
A fully equipped and adjustable manufacturing platform
to meet your needs
Production
Rapid Test LFIA
With a fully adjustable platform, BIOTEM can support its clients through the entire Lateral Flow test production process.
Lamination: Mastersheet/ Card Assembly
- Solid Phases: Nitrocellulose membrane, Sample Pad, Conjugate Pad, Absorbant Pad, etc.
- Features: 300 to 800 mm long / 60 to 90 mm wide
- Dry Room (humidity <30%)
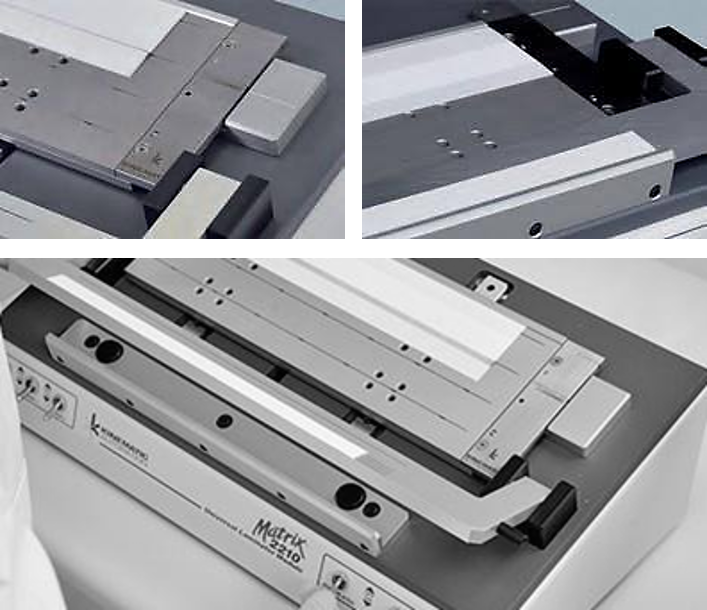
 Coating, Spraying and Dispensing
Coating, Spraying and Dispensing
- Biological agents preparation (conjugated antibodies, buffers, etc.)
- Wet room with a controlled environment (humidity >80%)
- BIODOT Systems
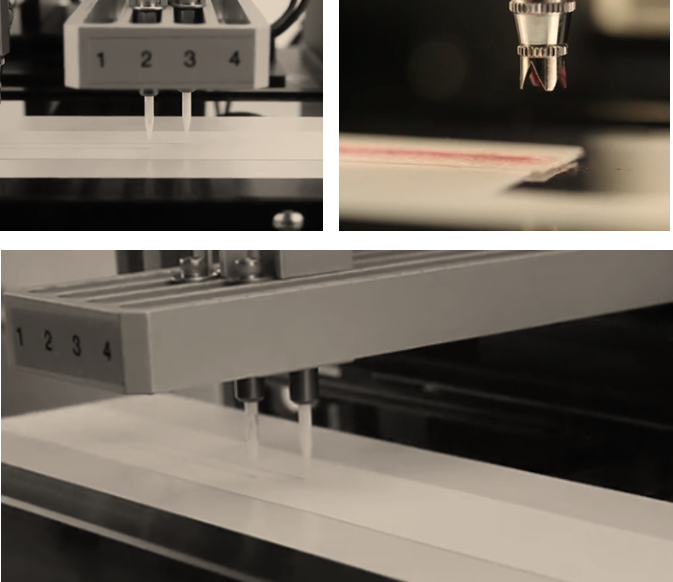
 Drying, Cutting and Packaging
Drying, Cutting and Packaging
- Dry room and et vacuum drying oven
- Automatic strip cutting
- Housing in cassette if needed
- Packaging in tubes or sealed sachets with desiccant.
Additional
Services
BIOTEM also offers its clients “Fill and Finish” services, to deliver a finished product according to precise specifications and quality standards.
Formulation and Solution Distribution
- Fully equipped ISO 5 / ISO 7 clean rooms
- Automated process
- Bottling and labelling

Kits Assembly / Packaging
- Dedicated and equipped room
- Packaging proposal / boxes, aluminum pouches and supports
- Label printing or client graphics
Storage (on request)
- Dedicated and securized building
- Temperature-controlled refrigerated area
- Storage of biologicals and raw materials at suitable and controlled temperatures (room temperature; -20°C or -80°C)
Production
ELISA test (96-well plates)
BIOTEM has the teams and equipment required for all stages of the ELISA kit production value chain.

Plate Coating
- Wet room with a controlled environment (humidity >80%)
- 96-well plate format
- Standard Process or with stabilization phase
 Plate Drying and Sealing
Plate Drying and Sealing
- Dry room optimized for some fragile biologicals
- Vacuum chambers
- Heat sealing machines
Additional
Services
BIOTEM also offers its clients “Fill and Finish” services, to deliver a finished product according to precise specifications and quality standards.

Formulation and Solution Distribution
- Fully equipped ISO 5 / ISO 7 clean rooms
- Automated process
- Bottling and labelling

Kits Assembly / Packaging
- Dedicated and equipped room
- Packaging proposal / boxes, aluminum pouches and supports
- Label printing or client graphics
Storage (on request)
- Dedicated and securized building
- Temperature-controlled refrigerated area
- Storage of biologicals and raw materials at suitable and controlled temperatures (room temperature; -20°C or -80°C)

To access the document, fill the form