Development of ELISA and Lateral Flow Rapid Tests
Immunoassays allow the detection and quantification of various targets in different types of samples. They are based on the recognition between one or more antibodies and an antigen.
BIOTEM proposes contracts with guaranteed results for the development and validation of ELISA and LFIA tests (rapid tests, Lateral Flow Immunoassay).
The company offers also a fully integrated, equipped and scalable manufacturing platform for the industrial production of kits.
Thanks to the ISO 13485:2016 certification (see Quality Policy), the tests developed by BIOTEM can be used as in vitro diagnostic medical devices (IVD) and in all types of applications:
Human health, Animal health, Industrial quality control, Agriculture, Biosecurity, Drugs, Environment, etc.

1. Project specifications
For each project, our team explores with the client the challenges of the project and defines the specifications. At the end of this phase BIOTEM recommends one or several strategies for the development.
- Analyte to be detected/ quantified
- Type of test: LFIA (rapid test) or ELISA
- Performances of the test
- Matrix (blood, urine, saliva, etc.)
- Available material (antibodies, antigens, samples, etc.)
2. Feasibility
The feasibility study is the first experimental step of the development process during which BIOTEM evaluates and selects the best components available (antibodies, antigens, membranes, conjugates, etc.). The company has expertise in the high quality generation antibodies (mice / rats, rabbits, etc.), proprietary technologies for antigen conjugation and optimized tracers. BIOTEM has the ability to integrate, from the early stages of antibody development, specific screenings to identify the best functional antibodies in the final format. BIOTEM initiates the development of different prototypes according to the development strategy previously defined.
Finally, BIOTEM proposes a development contract with guaranteed results or resource commitments (see below).
3. Assay Development
This development phase will depend mainly on the results obtained during the feasibility study. Within the framework of a resource commitment, BIOTEM proposes the implementation of test sessions with the evaluation of several parameters according to an experimental plan validated with the CLIENT. Regular reports are sent out to communicate the results and recommendations of our teams. Depending on the final application, this phase can be carried out to ISO 9001 and/or ISO 13485 (DMDIV) standards.
BIOTEM produces and sends to the client a batch of the prototype(s) developed for evaluation and/or validation.
4. Assay Optimization
BIOTEM optimizes prototypes initially developed during the feasibility study. During this phase several validations of the prototypes are undertaken in order to ensure that the development is in line with the project specifications.
BIOTEM produces and sends to the client a batch of the prototype(s) developed for evaluation and/or validation.
5. Production & Industrialization
BIOTEM and the client jointly carry the formal evaluation (laboratory or field trials) of the performance and the several characteristics of the test. This validation phase is performed to meet the client’s specifications :
- Limit of detection
- Linearity / Parallelism
- Specificity
- Precision
- Inter- and Intra-assay reproducibility
- Robustness
- Stability studies of the test and its components
To complete the project, BIOTEM proposes several options to the client :
- Transfer of technology to the client or client’s manufacturer
- Industrialization and batch production
BIOTEM offers a fully integrated and scalable manufacturing platform for the industrial production ELISA and Lateral Flow (strips/ cassettes) kits. Learn more
Immunoassay Development Platform
Which Formats?
Presentation of
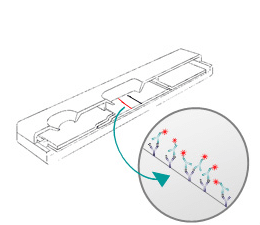
Rapid Test LFIA
Lateral Flow tests (immunochromatography system assays) are based on the migration of nano or micro particles on strips for analytes detection in several areas. These tests, strips or cassettes (example: the pregnancy test), are also called Point of Care tests (POCT) and are widely used in first-line emergency tests performed at the patient’s bedside or directly in the field.
Prototype Development:
- Mastersheet/ Card Lamination
- Biological Preparation (antibody conjugated to tracers, buffers, etc.)
- Component Preparation
- Spraying, Dispensing, Drying and Cutting
- Cutting and housing (strips & cassettes)
- Shipment of prototypes
Results
Commitments
Both the client and BIOTEM define together a contract containing :
- Success criteria definition
- Method of validation
- “Success fee” clause (payment in case of success only)
Guaranteed results contracts are the best commitments that BIOTEM is proud to propose to its clients which reflects BIOTEM’s excellence..

Resources
Commitments
In occasional cases, it will not be feasible to offer results commitments (mostly when success criteria cannot be clearly defined). The test development will then consist of a series of intermediary phases (each with a set duration) during which BIOTEM allocates especially dedicated resources.
- Intermediary phases reports including the results
- Development work suggestions.
After a discussion with BIOTEM, the client decides on the continuation of the project (GO/no GO).
Presentation of
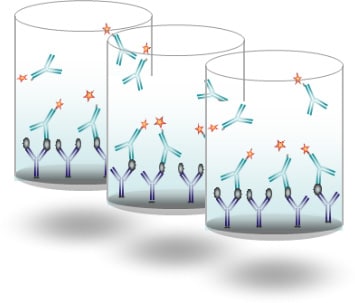
ELISA test
Principally used for R&D and for in vitro diagnostic, ELISA tests (plates) are enzymatic immunoassays for the multiplex detection and quantification of analytes in equipped laboratories.
Development and Industrial Production:
- Biological Preparation (antibody conjugation, buffers, etc.)
- Component Preparation
- Coating & Drying of Plates
- Shipment of prototypes
Results
Commitments
Both the client and BIOTEM define together a contract containing :
- Success criteria definition
- Method of validation
- “Success fee” clause (payment in case of success only)
Guaranteed results contracts are the best commitments that BIOTEM is proud to propose to its clients which reflects BIOTEM’s excellence.

Resources
Commitments
In occasional cases, it will not be feasible to offer results commitments (mostly when success criteria cannot be clearly defined). The test development will then consist of a series of intermediary phases (each with a set duration) during which BIOTEM allocates especially dedicated resources.
- Intermediary phases reports including the results
- Development work suggestions.
After a discussion with BIOTEM, the client decides on the continuation of the project (GO/no GO).


To access the document, fill the form