Quinthetic LibraryTM: Recombinant Antibodies from Synthetic Libraries
The total number of antibody specificities available to an individual’s immune system is known as the antibody repertoire, and in humans is at least 1011 .
(Source: Immunobiology: The Immune System in Health and Disease. 5th edition)
By choosing to introduce antibody variability both in the CDR (different lengths and ratios of specific amino-acids at optimized positions) and in the Vernier residues (a key subset of 3D-positioning specific amino-acids), BIOTEM designing scientists T. Pelat and A. Guellouz allow to potentiate paratope diversity both in CDR sequences and their orientations. This strategy based on ‘‘quintessence of variability’’ (by opposition to purely random variabilities) generates a new type of extremely efficient synthetic library that we call Quinthetic LibraryTM.
Where diversity really matters
VHH Quinthetic LibraryTM: Humanized VHH Library
scFv Quinthetic LibraryTM: Fully Human scFv Library
This ready-to-use offer completes the services for the generation of immune libraries focused on immunogens of interest. It can be an alternative to immunizations in the case of toxic, poorly immunogenic, highly conserved targets or targets that sometimes correspond to vital functions.
This solution also allows to obtain antibodies very quickly and at a lower cost, by saving the efforts and costs of production of large quantities of immunogens.
A service of sequence optimization / affinity maturation can be performed if needed. BIOTEM will also be able to propose a large panel of associated services in order to characterize and produce the antibody candidates in GMP-like conditions thanks to its recombinant antibody production platform.

Libraries developed with the support of La Region Auvergne-Rhône-Alpes.
General Advantages of Synthetic Libraries:
- Rapid (6 to 8 weeks)
- Animal-Free Technology
- Few Antigen Needed (< 150µg)
- Suitable for Non-Immunogenic and Toxic Targets
VHH
Overview
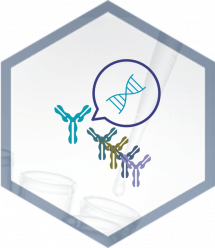
1. VHH Overview
A VHH (single-domain antibody or nanobody) is an antibody fragment naturally produced in camelids (llamas, dromedaries, etc.). Thanks to their small size (15 kDa), VHH can cross some physiological barriers and could be more effective in human and animal health. Moreover, unlike conventional IgG antibodies, VHH are able to bind to less exposed regions and cryptic epitopes with very high specificity and exceptional solubility, thus offering multiple advantages as therapeutic tools.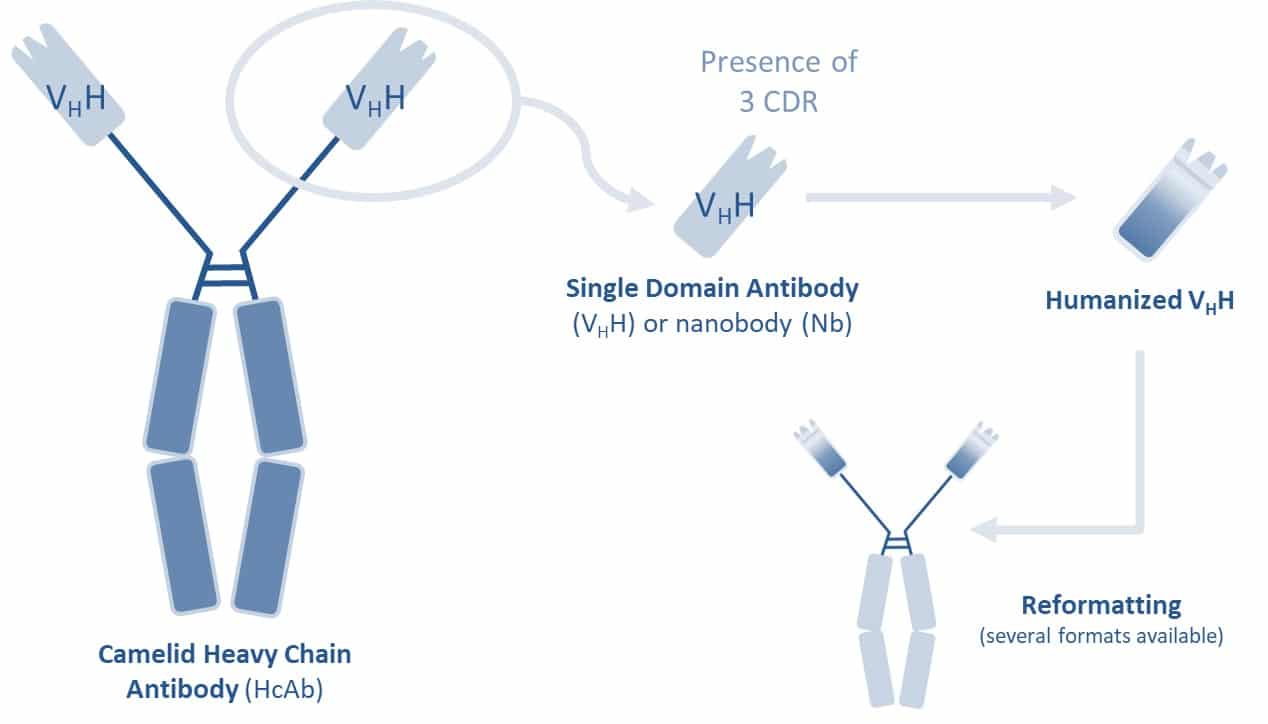
BIOTEM VHH Library
Advantages
2. BIOTEM VHH Library Advantages
BIOTEM proposes VHH Quinthetic LibraryTM, a synthetic library of humanized VHH. This library has many advantages compared to the libraries already available on the market. It has already been challenged on a large number of antigens (soluble protein, membrane, peptide, cell, etc.), with a success rate approaching 90%!
- Optimized design to have a very high stability (pH, temperature, protease), a focused diversity (high spot and anchoring residues), a better manufacturability (PTM)
- Suitable for all types of applications: from research (crystallography), to diagnostics (immunoassays) and to pre-clinical studies in therapeutics thanks to their pre-humanization (about 85%)
- Easy reformatting: with its unique chain, several possibilities of production in different recombinant formats (bivalent, tetravalent, etc.)
- Library Size > 3 x 109: allowing to optimize the chances of success in obtaining antibodies after screening
Screening
Phage Display
3. Screening by Phage Display
Depending on the target of interest and the CLIENT's criteria, the teams will propose an adapted high-throughput screening (or bio-panning) by phage display in order to identify the best candidates with direct access to the sequences.
- Selection of humanized VHH in a few weeks
- Standard or optimized screening according to client specifications
- Immediate and automatic access to sequences (single clones - redundancy analysis)
- Production, Purification and Characterization of candidates (reactivity, affinity, etc.)
At the end of this phase the client will have the opportunity to test the VHH and select the best candidates for large scale production and/or sequence optimization process.
Optimization
& Production
4. Optimization & Large Scale Production
According to the results obtained after screening, the CLIENT will have the possibility to select the best candidate to carry out a sequence optimization/affinity maturation (if necessary) and to launch a large scale production.
BIOTEM also has a panel of services of antibody engineering allowing to produce the VHH in different formats (VHH-Fc, bivalent, tetravalent, etc.) thanks to its platform of production of recombinant antibodies by transient transfection in CHO cells. Learn more
- Production from milligrams to grams
- Low endotoxin conditions (< 10 EU/mg ; < 1 EU/mg)
- Serum free system
- GMP-like grade
- Quality controls (concentration, purity, affinity, aggregation, stability, etc.).
scFv
Overview
1. scFv Overview
A scFv (single-chain variable fragment) is not really an antibody fragment but rather a fusion protein of VH and VL variable regions associated by a linker. Thanks to its size of about 25 kDa but also to its ease of expression and reformatting, the scFv can be used in different applications such as therapeutics with intracellular antibodies (intrabodies) or CAR-T cells expressing a scFv on their surface. scFv can also be used as a diagnostic tool, in particular with the possibility to conjugate it with different types of molecules or tracers (fluorescent molecule, enzyme, etc.).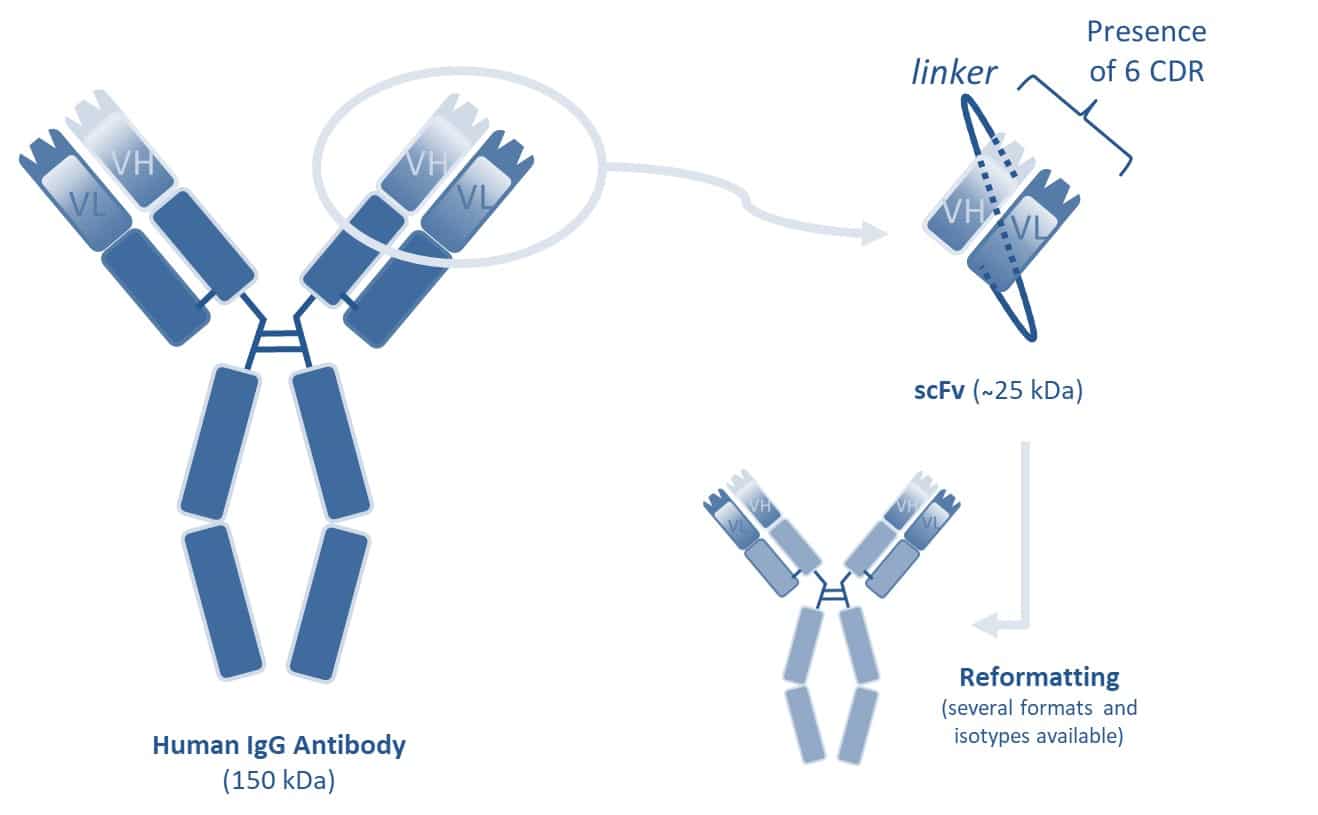
BIOTEM scFv Library
Advantages
2. BIOTEM scFv Library Advantages
BIOTEM proposes scFv Quinthetic LibraryTM, a synthetic library of fully human scFv. This library has many advantages compared to the libraries already available on the market.
- Optimized design with the selection of a highly stable scaffold and a strong focus on diversity (mutagenesis)
- Suitable for all types of applications: from research to diagnostic (immunoassays) and pre-clinical studies in therapeutics thanks to the 100% human format.
- Easy reformatting: many possibilities of production in different recombinant formats (scFv-Fc, dimeric scFv, IgG, etc.)
- Library Size ~1010: allowing to optimize the chances of success in obtaining antibodies after screening
Screening
Phage Display
3. Screening by Phage Display
Depending on the target of interest and the CLIENT's criteria, the teams will propose an adapted high-throughput screening (or bio-panning) by phage display in order to identify the best candidates with direct access to the sequences.- Selection of humanized scFv in a few weeks
- Standard or optimized screening according to client specifications
- Immediate and automatic access to sequences (single clones - redundancy analysis)
- Production, Purification and Characterization of candidates (reactivity, affinity, etc.)
Optimization
& Production
4. Optimization & Large Scale Production
According to the results obtained after screening, the CLIENT will have the possibility to select the best candidate to carry out a sequence optimization/affinity maturation (if necessary) and to launch a large scale production. BIOTEM also has a panel of services of antibody engineering allowing to produce the scFv in different formats (scFv-Fc, bivalent scFv, Full IgG, etc.) thanks to its platform of production of recombinant antibodies by transient transfection in CHO cells. Learn more- Production from milligrams to grams
- Low endotoxin conditions (< 10 EU/mg ; < 1 EU/mg)
- Serum free system
- GMP-like grade
- Quality controls (concentration, purity, affinity, aggregation, stability, etc.).


To access the document, please fill the form