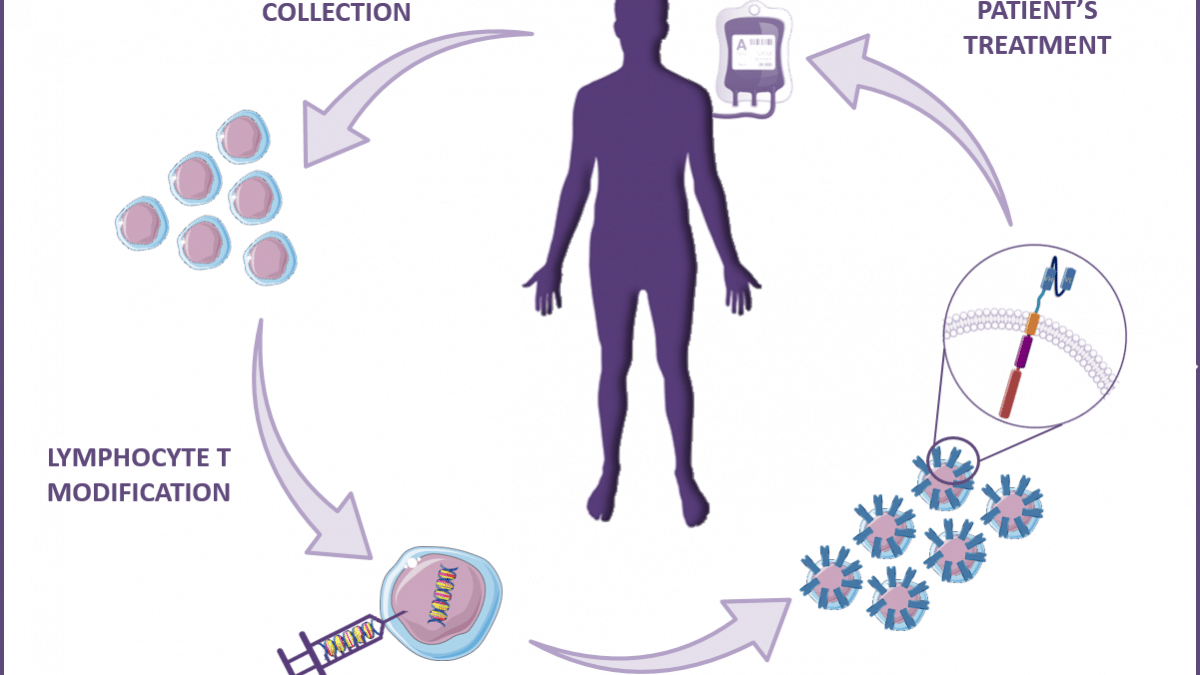
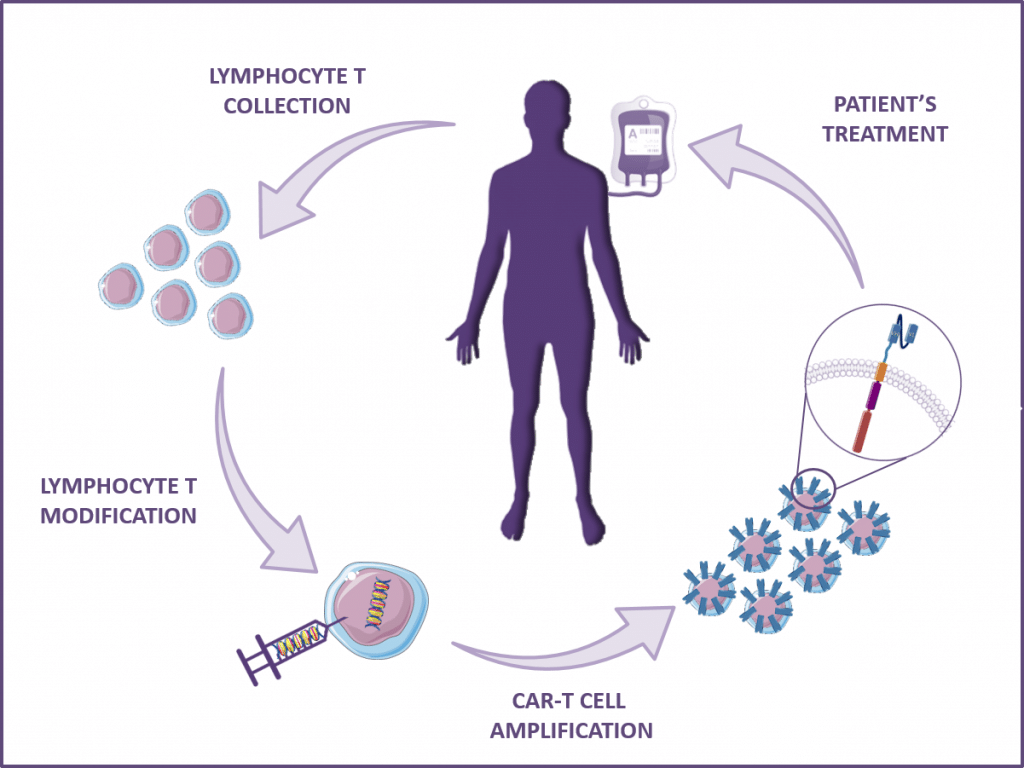
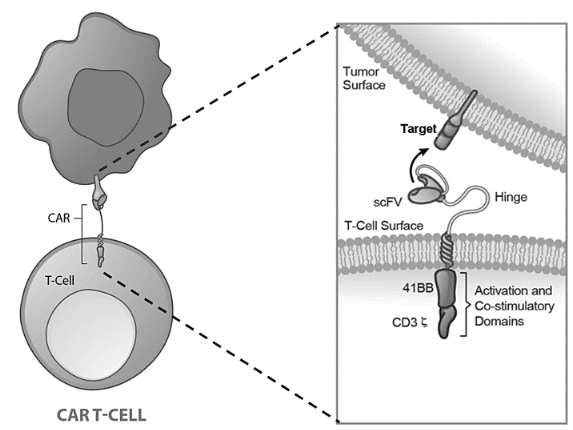
Other applications for scFv or VHH / nanobody
Due to its small size (25kDa), scFv can be easily expressed in cells. Furthermore, the recombinant format allows for easy insertion of sequences to address the antibody to specific cellular compartments. Then, the scFv can neutralize/block and also monitor an intracellular marker, which raises the possibility of innovative therapies.
In addition, Full-length monoclonal antibodies are not suitable for certain pathologies such as neurodegenerative diseases where the Blood Brain Barrier (BBB) prevents their passage. Indeed, their size is too large (around 150 kDa) which does not allow them to cross it. The recent emergence of small antibodies is promising in diagnosis, in veterinary health and more recently in human health as well, especially in the fight against brain disorders such as Alzheimer’s disease and Parkinson’s disease. For example, a patented anti-Tau VHH (15kDa), binds to the pathological forms of the Tau protein in Alzheimer’s disease (AD) [3]. We can also mention two other VHH – effective in AD too – which are specific to protofibrils [4] and Aβ40 oligomers [5].
The size of the scFv and its expression as recombinant antibodies also allows the possibility of generating fusion proteins with different types of molecules (enzyme, fluorescent molecules, etc.). This makes the antibody fragment an excellent tool for immunoassay development.
The low molecular weight and good water solubility of VHH allow them to diffuse well through biological barriers such as the Blood Brain Barrier (BBB) and to reach intracellular components. VHH are thus a tool of choice in the diagnosis of neurodegenerative pathologies such as brain imaging techniques: VHH are usually coupled with a radioelement (for example 11C or 18F isotopes) and used as radio-pharmaceutical agents. Moreover, due to these physical and chemical characteristics, VHH are very rapidly eliminated molecules (fast renal clearance) [6].